Hyperkalemia Risk Calculator
Risk Factors Assessment
Enter patient details to calculate hyperkalemia risk from combined ACE inhibitor and potassium-sparing diuretic therapy.
- Check potassium within 2 weeks of starting therapy
- Recheck potassium every 1-3 months
- Monitor kidney function
When doctors prescribe an ACE inhibitors is a class of drugs that block the conversion of angiotensin I to angiotensin II, lowering blood pressure and easing heart failure. At the same time, many patients need a potassium-sparing diuretics to prevent fluid overload without dumping potassium in the urine. Putting these two together can feel like a perfect match-both protect the heart and kidneys-but the combo also creates a perfect storm for hyperkalemia. This article breaks down why, who should worry most, and what you can do to stay safe.
Why the Combination Raises Alarm
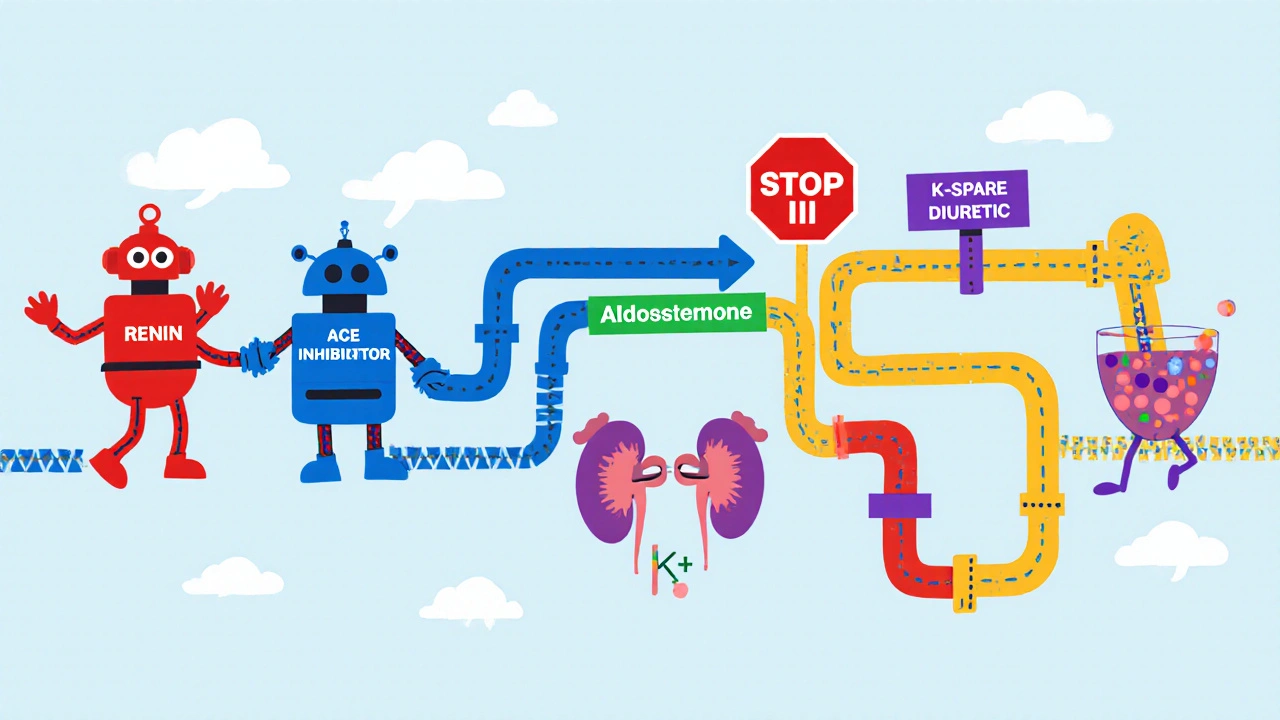
Both drug families hit the same hormonal pathway: the renin‑angiotensin‑aldosterone system (RAAS). ACE inhibitors shut down angiotensin II production, which in turn slashes aldosterone release. Without aldosterone, the kidneys lose a key signal to push potassium out in the collecting ducts. Aldosterone is a hormone that normally tells the kidney to re‑absorb sodium and excrete potassium. When you add a potassium‑sparing diuretic, you’re either blocking aldosterone’s receptor (as with spironolactone) or directly inhibiting the epithelial sodium channel (ENaC) with drugs like amiloride). The result is a ‘double hit’ on potassium elimination, and serum levels can climb quickly.
How the Mechanisms Overlap
Here’s a quick walk‑through of the two‑step process:
- ACE inhibition → ↓ Angiotensin II → ↓ Aldosterone → reduced activation of ENaC.
- Potassium‑sparing diuretic → blocks aldosterone receptor (spironolactone, eplerenone) or blocks ENaC directly (amiloride, triamterene) → further ↓ potassium excretion.
The synergy explains why studies repeatedly report a 3‑ to 5‑fold rise in hyperkalemia risk when the drugs are used together. For example, the REIN study showed hyperkalemia jumping from 4.2 % on ACE inhibitors alone to 18.7 % when spironolactone was added.
Who Is Most at Risk?
Not everyone who takes these meds will develop high potassium, but certain red flags dramatically increase the odds:
- eGFR (estimated glomerular filtration rate) < 60 ml/min/1.73 m² - the lower the number, the worse the kidney’s ability to clear potassium.
- Baseline serum potassium > 4.5 mmol/L.
- Diabetes mellitus, especially if renal function is already compromised.
- Congestive heart failure or recent myocardial infarction.
- Concurrent use of other potassium‑raising agents (e.g., NSAIDs, trimethoprim).
Using a simple scoring system, Cleveland Clinic researchers assign points to each factor. A total of 4 or more signals high risk and triggers intensive monitoring.
Monitoring and Prevention Strategies
Guidelines from the AHA (2016) and KDIGO (2021) converge on a few core actions:
- Check serum potassium and creatinine within 1-2 weeks of starting an ACE inhibitor, especially if eGFR is < 60.
- Repeat labs every 3-6 months for stable patients; increase frequency (monthly or weekly) for those with CKD stage 3‑4.
- Consider starting the combination at half the usual dose. Titrate up only after confirming potassium stays ≤ 5.0 mmol/L.
- Educate patients about dietary potassium: limit bananas, oranges, potatoes, and tomatoes to keep intake < 75 mmol/day.
- When possible, add a thiazide or loop diuretic to the regimen. These drugs pull potassium out of the body and have been shown to lower hyperkalemia risk by about a third.
For high‑risk individuals, the Cleveland Clinic checklist recommends a baseline measurement of creatinine (target < 1.5 mg/dL) and a repeat potassium test within 7 days of any dose change.
Managing an Episode of Hyperkalemia
If a patient’s potassium climbs above 5.0 mmol/L, act early:
- Review recent labs, medication changes, and diet.
- Temporarily hold the potassium‑sparing diuretic.
- Reduce the ACE inhibitor dose by 25‑50 %.
- Consider adding a non‑potassium‑sparing diuretic (hydrochlorothiazide 12.5‑25 mg daily) to bring potassium down 0.5‑1.0 mmol/L within two weeks.
- If potassium exceeds 5.5 mmol/L, add a binder such as patiromer (Veltassa) or sodium zirconium cyclosilicate (Lokelma). Clinical trials show these agents lower potassium by ~1 mmol/L in 48 hours and let patients stay on their life‑saving RAAS blocker.
- In severe cases (> 6.0 mmol/L) with ECG changes, emergency measures (IV calcium, insulin‑glucose, beta‑agonists) are required and nephrology should be consulted.
After stabilization, re‑evaluate the overall regimen. Switching from an ACE inhibitor to an ARB can shave roughly 18 % off the hyperkalemia risk, according to Sadjadi’s 2009 data.

Emerging Therapies and Future Directions
New tools are reshaping how clinicians handle potassium:
- SGLT2 inhibitors (e.g., dapagliflozin) have cut hyperkalemia incidence by about a third in CKD patients on ACE inhibitors.
- Digital health apps that log dietary potassium are reducing episodes by roughly 27 % in recent pilot studies.
- Point‑of‑care potassium meters are in phase III trials and could enable same‑day dose adjustments.
- Genotype‑guided dosing (especially WNK1 polymorphisms) may allow personalized risk scores by 2026.
While these innovations are promising, the core principle remains the same: know the drugs, know the patient, and monitor closely.
Risk Comparison Table
| Regimen | Incidence of K⁺ >5.0 mmol/L | Typical Odds Ratio vs. ACE I alone | Key Mitigation |
|---|---|---|---|
| ACE I alone | 11 % | 1.0 (reference) | Standard monitoring |
| ACE I + Spironolactone | 19.7 % | 3‑5× | Lower doses, add thiazide, binders if needed |
| ACE I + Loop Diuretic | 6 % | 0.66 (protective) | Routine labs |
| ARB alone | 9 % | 0.78 | Same as ACE I |
| ARB + Eplerenone | 16 % | 2.5× | Half dose start, monitor eGFR |
Bottom Line Checklist
- Identify high‑risk patients (eGFR < 60, diabetes, heart failure).
- Start combination therapy at ≤ 50 % of full dose.
- Check potassium & creatinine within 1‑2 weeks, then every 3‑6 months.
- If K⁺ > 5.0 mmol/L, hold potassium‑saver, reduce ACE I, add a thiazide or loop.
- Consider patiromer or sodium zirconium cyclosilicate for persistent elevation.
- Educate patients on dietary potassium and medication timing.
- Re‑assess after any dose change, acute illness, or new drug added.
What potassium level is considered dangerous?
A serum potassium above 5.5 mmol/L warrants intervention, and levels over 6.0 mmol/L can cause life‑threatening heart rhythm problems.
Can I safely take a potassium‑sparing diuretic with an ACE inhibitor?
Yes, but only if you’re low‑risk and you follow strict lab monitoring. Start at reduced doses and consider adding a thiazide to offset the potassium rise.
How often should labs be drawn after I start this combo?
Check within 1‑2 weeks, then repeat at 1 month, 3 months, and every 6 months thereafter if stable. High‑risk patients need monthly checks for the first 3 months.
Are potassium binders safe for long‑term use?
Both patiromer and sodium zirconium cyclosilicate have been studied for up to two years with few serious side effects. They’re especially useful when you can’t discontinue the ACE inhibitor.
What dietary changes help lower potassium?
Aim for < 75 mmol of potassium per day. Limit high‑potassium foods like bananas, oranges, potatoes, tomatoes, and processed foods with added potassium chloride.


Seriously, prescribing ACE inhibitors together with potassium‑sparing diuretics without a strict monitoring plan is a reckless gamble. It’s like handing a teenager a loaded gun and hoping they won’t pull the trigger. The RAAS blockade already reduces aldosterone, and adding a spironolactone just shuts the potassium‑excreting system down completely. You end up with a perfect storm of hyperkalemia that can turn fatal in days. If doctors aren’t willing to run labs every week, they should think twice before using this combo. The risks far outweigh any modest blood‑pressure benefit for most patients.
First off, it’s great that you’re looking into this. The key is balance: start low, monitor labs, and involve the patient in dietary choices. If you catch a rise to 5.0 mmol/L early, a simple dose tweak or adding a thiazide can prevent escalation. Education on potassium‑rich foods makes a huge difference, and most patients appreciate the proactive approach. Keep an eye on eGFR trends; a steady decline signals you need tighter control. Overall, with careful titration, many patients stay on both meds safely.
Wow, this article really breaks down the double‑hit mechanism, doesn’t it? You’ve got the ACE‑I cutting down angiotensin II, then the potassium‑saver blocking aldosterone receptors – it’s almost poetic, yet terrifying for potassium levels! I love the checklist approach, especially the 1‑2‑week lab window; it gives clinicians a clear action plan. And the dietary tips? 💡 Those “bananas, oranges, potatoes” reminders are spot‑on for patient education. Overall, a solid, practical guide that balances theory with bedside relevance.
Good info. Keep labs tight. Watch eGFR. Reduce dose if needed.
Let’s keep the momentum going, folks! 🌟 The combo can work if we treat it like a team sport – every player (doctor, nurse, patient) watches the scoreboard (labs) and makes quick passes (dose tweaks). Remember, a half‑dose start is like a warm‑up stretch before the big lift. Celebrate small wins – a stable potassium reading is a victory dance waiting to happen! Keep sharing tips and stories; together we can turn this risky duo into a well‑managed partnership.
I think the doc is missng the point, this combo is a disasterr, patients get hyperkalaemia like never before. You cant just throw a thiazide in and hope it fixes everything, that's sooo lazy. Ppl need real protocols not just "do it and see". If you dont monitor weekly, you might as well be playing Russian roulette with their hearts.
Quick tip: start spironolactone at 12.5 mg and ACE‑I at half the usual dose. Check K+ and creatinine in 7‑10 days, then adjust. Adding a low‑dose loop can keep K+ down while preserving the protective benefits. Simple and effective.
The pharmacodynamic interplay between ACE inhibitors and potassium‑sparing diuretics represents a quintessential exemplar of synergistic nephro‑cardiovascular modulation, wherein the attenuation of angiotensin‑II synthesis precipitates a consequent diminution of aldosterone secretion, thereby attenuating the distal nephron’s capacity for sodium reabsorption and concomitant potassium excretion. When a mineralocorticoid receptor antagonist such as spironolactone is introduced, the pharmacologic blockade ensues at a downstream node, obviating aldosterone‑mediated transcriptional upregulation of the epithelial sodium channel. Consequently, the net effect is a compounded reduction in potassium clearance, manifesting as a clinically salient elevation in serum K+. Empirical data from the REIN cohort elucidate a quintupled incidence of hyperkalemia under combined therapy, underscoring the imperative for rigorous surveillance. Moreover, the risk stratification schema delineated by Cleveland Clinic incorporates renal function metrics, baseline serum potassium, comorbid diabetes, and concurrent nephrotoxic agents, each contributing weighted points toward a composite risk index. The operative threshold of four points predicates intensified monitoring protocols, including biweekly serum chemistries during titration phases. Therapeutically, a judicious titration strategy-initiating at 50 % of target dose-mitigates precipitous potassium surges while preserving hemodynamic benefits. Adjunctive loop or thiazide diuretics can be employed to augment urinary potassium excretion, thereby rebalancing homeostasis. In refractory cases, potassium binders such as patiromer or sodium zirconium cyclosilicate afford a mechanistic avenue to sequester luminal potassium, facilitating continuation of renin‑angiotensin system inhibition. It is paramount to acknowledge that emergent modalities, including SGLT2 inhibitors, have demonstrated ancillary potassium‑lowering effects, potentially synergizing with existing regimens. Nonetheless, clinical prudence dictates a patient‑centric approach, integrating renal function trajectories, medication adherence, and dietary potassium intake. The overarching therapeutic ethos remains anchored in the dual objectives of preserving cardiovascular integrity while averting iatrogenic electrolyte derangements. Continuous quality improvement initiatives and integration of point‑of‑care potassium testing may further refine risk mitigation strategies in the near future.
They dont want you to know that pharma pushes these combos to sell more drugs the labs are just a distraction they keep you in the dark
In practice, initiating the combination at reduced dosages and scheduling potassium checks at 7‑day intervals has markedly reduced adverse events. Patients also respond positively when we provide clear dietary guidance, reinforcing adherence. 😊
Oh great, another perfect combo for hyperkalemia – just what we needed.
Actually the risk is overblown i have seen no issues.
In my clinic we never had a problem because we keep the doses low and slap a loop on top, no need for all this hype.
Can we talk about the sheer drama of watching potassium levels dance on the edge of danger? One minute you’re calm, the next a lab result screams “5.6!” and your heart does a flip‑flop. It’s like a soap‑opera where the villain is a simple mineral, and the hero is a diuretic that shows up just in time to save the day. The tension, the relief… I’m living for this rollercoaster!