Ever picked up a prescription and noticed the pill looks different-same size, same color, same imprint-but the name on the bottle isn’t the one your doctor wrote? You might think you’re getting a cheaper, lower-quality version. But what if that pill is actually exactly the same as the brand-name drug you’ve been taking? That’s the reality with authorized generics.
What Exactly Is an Authorized Generic?
An authorized generic is a brand-name drug sold without the brand name on the label. It’s not a copy. It’s not a knockoff. It’s the exact same pill, capsule, or injection that comes in the original box-with the same active ingredients, same inactive ingredients, same shape, same coating, same manufacturing process. The only difference? No brand logo. No fancy packaging. Just the drug itself.
The U.S. Food and Drug Administration (FDA) defines it clearly: an authorized generic is produced under the same New Drug Application (NDA) as the brand-name version. That means it doesn’t go through the separate approval process that traditional generics do. It’s not just bioequivalent-it’s identical. If your doctor prescribes Lipitor, and you get an authorized generic, you’re getting the exact same formula Pfizer made, just without the Lipitor name on it.
How Is It Different From a Regular Generic?
This is where things get confusing. Most people think all generics are the same. They’re not.
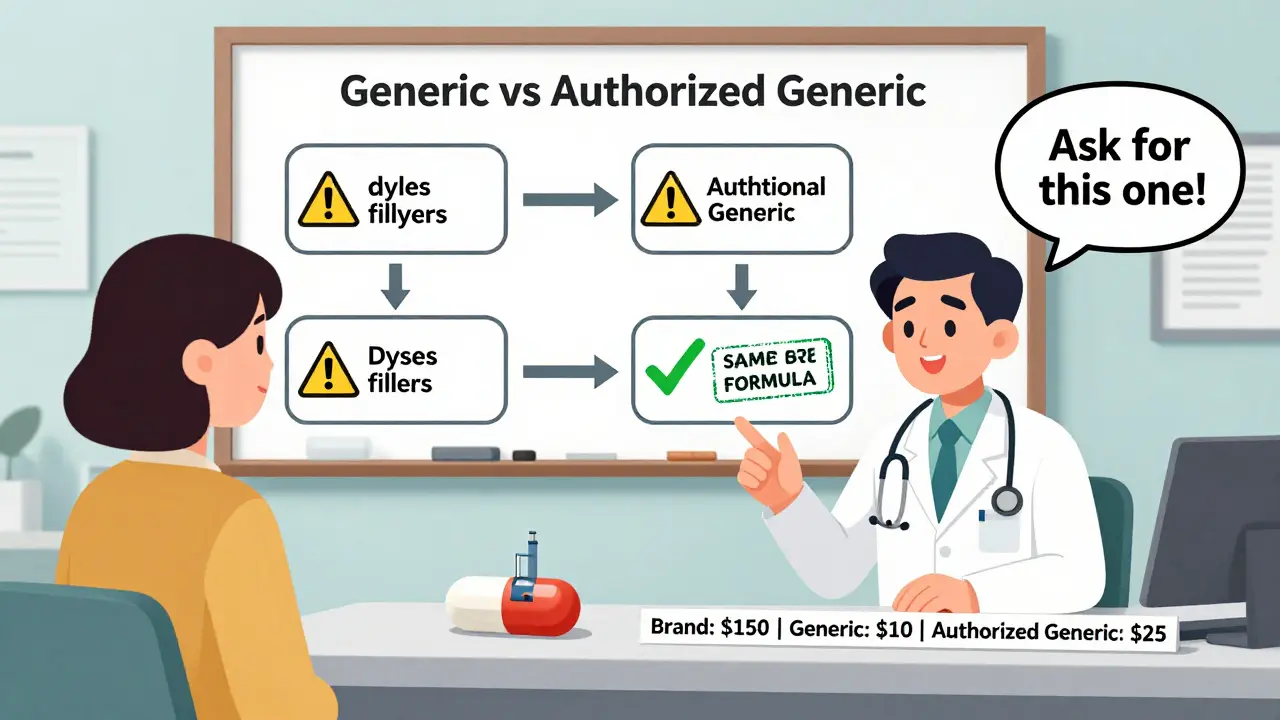
Traditional generics must prove they’re bioequivalent to the brand drug. That means they deliver the same amount of active ingredient into your bloodstream within a certain range-usually within 80% to 125% of the brand. That sounds close, right? But here’s the catch: traditional generics can have different inactive ingredients. Think fillers, dyes, preservatives, coatings. These don’t affect how the drug works, but they can affect how your body reacts to it.
For most people, this doesn’t matter. But for those with allergies, sensitivities, or digestive issues, even small changes in inactive ingredients can cause problems. One patient might switch from a brand to a generic and start having stomach upset or a rash. That’s often due to a dye or binder that wasn’t in the original.
Authorized generics don’t have that problem. They use the same inactive ingredients as the brand. No substitutions. No surprises. If you’ve had a bad reaction to a traditional generic before, an authorized generic might be the solution.
Why Do Brand Companies Sell Authorized Generics?
It seems counterintuitive. Why would a company that spent billions developing a drug turn around and sell the exact same thing for less?
The answer is competition. When a brand drug’s patent expires, other companies can legally make generics. To protect their market share, brand manufacturers sometimes launch their own authorized generic. It’s a smart business move: they get to keep some of the revenue from the generic market instead of losing it all to competitors.
But there’s a downside. Authorized generics are often priced higher than traditional generics. While a regular generic might cost 80-85% less than the brand, an authorized generic might only be 15-20% cheaper. That’s because the brand company still controls production and pricing. So while you’re getting a perfect match, you’re not always getting the best deal.

Are Authorized Generics Safe?
Yes. Absolutely.
The FDA says authorized generics are therapeutically equivalent to their brand-name counterparts-and they mean it. Since they’re made under the same NDA, with the same formula, same factory, same quality controls, there’s no difference in safety or effectiveness.
A 2018 study analyzing over 5,000 patients switching from brand drugs to generics found no meaningful difference in hospital visits, emergency room trips, or medication adherence between those who took authorized generics and those who stayed on the brand. The only slight outlier? A small increase in ER visits for authorized generics, but researchers believe that had more to do with patient confusion than drug performance.
Experts like Dr. Choudhry from Harvard Health confirm: authorized generics have zero variation in active ingredient levels. Traditional generics can vary by up to 20% (though in practice, it’s usually closer to 4%). Authorized generics? Zero. They’re the same batch, just repackaged.
Why Don’t More People Know About Them?
Because the system isn’t designed to make them obvious.
Pharmacists are trained to substitute generics when allowed by law. But they don’t always distinguish between traditional generics and authorized generics. If your prescription says “Lipitor,” and the pharmacy gives you a white pill labeled “atorvastatin,” you might assume it’s a regular generic. But it could be an authorized generic-same as the brand, just unlabeled.
And patients? Most don’t ask. They assume all generics are equal. A 2023 survey by Pharmacy Times found that nearly 30% of patients question a switch to an authorized generic, even though it’s identical to what they were taking before.
It’s not the pharmacist’s fault. It’s the system. Labels don’t say “authorized generic.” Insurance formularies don’t differentiate. Even doctors often don’t know unless they check.
How to Get an Authorized Generic
If you want one, you have to ask. Here’s how:
- Ask your doctor if they’re open to prescribing an authorized generic. Some will write “dispense as written” (DAW) on the prescription to block substitutions. Others will let you choose.
- When you get to the pharmacy, ask the pharmacist: “Is this an authorized generic?” They can check the label or contact the distributor.
- Look at the label. Authorized generics often have the manufacturer’s name (like Pfizer or Teva) and the drug name (e.g., “atorvastatin calcium”), but no brand name like “Lipitor” or “Zoloft.”
- Check your insurance formulary. Some plans cover authorized generics at the same tier as traditional generics. Others put them closer to brand pricing.
Some patients with allergies or autoimmune conditions specifically request authorized generics because they’ve had reactions to inactive ingredients in traditional generics. The American Academy of Allergy, Asthma & Immunology recommends this for patients with known sensitivities.
What About Cost?
Cost varies. Authorized generics are usually cheaper than the brand, but not always cheaper than traditional generics.
For example, a 30-day supply of brand-name Zoloft might cost $150. A traditional generic sertraline might cost $10. An authorized generic sertraline? Maybe $25. That’s still a savings, but not as big.
GoodRx data from 2023 shows that while traditional generics are typically 80-85% cheaper than brand drugs, authorized generics average only 15-20% off. That’s because the original manufacturer controls the price. They’re not trying to undercut the market-they’re trying to keep some of it.
But here’s the twist: sometimes, authorized generics are the cheapest option. If the brand has multiple generic competitors, the authorized version might be priced lower than the others to stay competitive. Always compare prices at your pharmacy.
What’s the Future of Authorized Generics?
The market for authorized generics is growing. As of 2023, the FDA listed over 150 authorized generics across 55 different drugs. Most are in chronic conditions-high blood pressure, cholesterol, depression, diabetes-where patients take the same pill every day for years.
But there’s pressure. Critics say brand manufacturers use authorized generics to delay true generic competition. By launching their own version, they can keep prices high and reduce the incentive for other companies to enter the market.
The Affordable Prescriptions for Patients Act of 2023 proposed rules to limit this practice. The FDA is also considering requiring authorized generics to be listed in the Orange Book-the official guide to drug equivalence-so patients and doctors can see them clearly.
For now, they’re here to stay. And for the right patient, they’re the best option.
Bottom Line
Authorized generics aren’t a compromise. They’re the real thing-same drug, same factory, same formula, just without the brand name. If you’ve had issues with traditional generics, or if you want the exact same medication you’ve been on for years, ask for one. You might save money. You might avoid side effects. And you’ll know you’re getting exactly what your doctor prescribed.
Are authorized generics as safe as brand-name drugs?
Yes. Authorized generics are made under the same FDA-approved New Drug Application (NDA) as the brand-name drug. They use the same active and inactive ingredients, come from the same manufacturing facility, and follow the same quality controls. The FDA confirms they are therapeutically equivalent-no difference in safety or effectiveness.
Can I trust an authorized generic if it looks different from my brand drug?
Absolutely. The appearance of the pill-color, shape, imprint-may change if the manufacturer is different, but the drug inside is identical. Authorized generics are the exact same product as the brand, just packaged without the brand name. If you’re unsure, ask your pharmacist for the manufacturer name or check the FDA’s list of authorized generics.
Why is my authorized generic more expensive than the regular generic?
Because it’s still made by the original brand company or its affiliate. They control the pricing and often set it higher than traditional generics to maintain revenue after patent expiration. While traditional generics compete on price, authorized generics are more about market control. Always compare prices-sometimes the regular generic is still cheaper.
Do insurance plans cover authorized generics?
Yes, but coverage varies. Many plans treat authorized generics the same as traditional generics and place them on the lowest cost-sharing tier. Others may price them closer to the brand. Always check your plan’s formulary or call your insurer to confirm your out-of-pocket cost before filling the prescription.
How do I know if my prescription is an authorized generic?
Look at the label. If it lists the generic name (like “atorvastatin”) and the manufacturer’s name (like Pfizer or Teva) but no brand name (like “Lipitor”), it’s likely an authorized generic. You can also ask your pharmacist directly: “Is this an authorized generic?” They can check the distributor information or contact the wholesaler for confirmation.


Just switched to an authorized generic for my blood pressure med last month and honestly? No difference at all. My doctor never mentioned it, but the pharmacist slipped me a note and I asked. Saved me like $40 a month and my heart still works the same. Sometimes the system actually works
Wait-so you’re telling me the white pill labeled ‘atorvastatin’ that I’ve been taking for two years… is literally the same as Lipitor? No joke? I’ve been paranoid this whole time thinking the pharmacy was cutting corners. My anxiety dropped more than my cholesterol did. Thanks for clarifying. I feel like an idiot now.
Of course the big pharma boys want you to think this is some kind of miracle. They’re not giving you a better deal-they’re keeping you hooked. The FDA? A puppet. The Orange Book? A joke. They let the same company make the brand and the ‘generic’ so they can charge whatever they want and pretend they’re helping you. This isn’t transparency-it’s manipulation dressed up as choice
OMG I KNEW IT 😱 They’re putting tracking chips in the pills!!! The authorized generics are the ones with the extra coating-same ingredients, same factory, but they’re monitoring your heartbeat through your tongue. I saw it on a forum. Don’t take them. I’m not even joking.
Let me get this straight-Big Pharma makes the drug, then sells the exact same pill under a different label to screw over the real generics? That’s not capitalism. That’s a crime scene with a lab coat. I’ve been on this stuff for 8 years and I just found out I was being played like a fiddle. My trust in medicine? Gone. Like, permanently.
Why are people so shocked? Of course the brand company makes an authorized generic. It’s called business. If you want the cheapest option, go for the real generic. If you want the exact same thing, pay a little more. It’s not a conspiracy-it’s economics. Stop acting like you’re being personally victimized.
My uncle in India takes the same medicine-authorized generic-and pays less than $2 a month. Here, we pay $25 and call it a bargain. We need real price control, not fancy labels. The science is the same. The greed is not.
Important note: Authorized generics are coded as ‘AB’ rated in the FDA’s Orange Book, same as traditional generics-but they’re often produced on the same line as the brand. That means batch consistency is near-perfect. If you’re sensitive to fillers (like lactose or FD&C dyes), this is your safest bet. Always check the manufacturer-Pfizer, Teva, and Mylan often make them.
Thanks for this. I’ve been switching generics every time my insurance changes and my stomach has been a mess. I just called my pharmacist and asked if my sertraline was an authorized generic. Turns out it was. No more bloating. No more weird rashes. Just… normal. I’m telling my whole family about this.
bloody hell i thought i was getting ripped off with the white pill… turns out i was just getting the real deal. my mate said i was being paranoid but turns out he’s the one who’s clueless. pharma companies are sneaky bastards but at least this one’s legit. cheers mate
Why do Americans care so much about pills? In my country, we just take what’s given. If it’s FDA-approved, it’s fine. You people turn medicine into a drama series. Just swallow the pill. The brand name doesn’t change the chemistry. You’re overthinking it.
It is of paramount importance to recognize that the regulatory equivalence of authorized generics, as codified under Title 21 CFR § 310.502, affirms their therapeutic interchangeability with the reference listed drug. The absence of brand nomenclature on the packaging does not constitute a material deviation in pharmacokinetic or pharmacodynamic parameters. Therefore, the clinical decision-making process should be informed by bioequivalence data, not marketing semantics.