When you pick up a generic pill at the pharmacy, you expect it to work just like the brand-name version. But how do regulators know it’s truly the same? The answer lies in two numbers: Cmax and AUC. These aren’t just lab terms-they’re the foundation of whether a generic drug gets approved and ends up on your shelf.
What Cmax and AUC Actually Measure
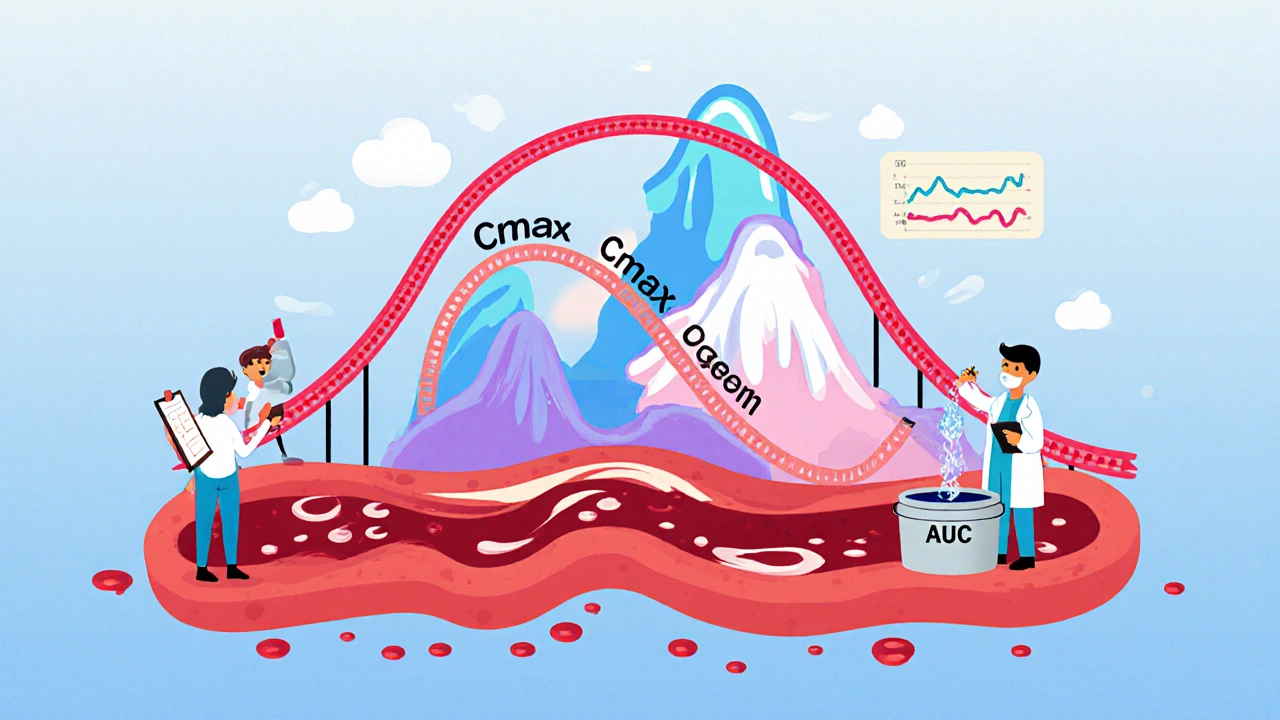
Cmax stands for maximum concentration. It’s the highest level a drug reaches in your bloodstream after you take it. Think of it like the peak of a rollercoaster-how high you go matters. If a drug needs to hit a certain concentration quickly to relieve pain or stop a seizure, Cmax tells you if the generic gets you there fast enough.
AUC, or area under the curve, measures total exposure. It’s the entire area under the graph of drug concentration over time. Imagine filling a bucket with water dripping from a hose. AUC tells you how much water ended up in the bucket, no matter how fast or slow it dripped. For drugs that work over hours-like antibiotics or blood pressure meds-total exposure is what matters most.
These aren’t theoretical. In a real bioequivalence study, researchers give volunteers the brand-name drug, then later give them the generic. They draw blood every 15 to 30 minutes for 24 to 72 hours, depending on the drug. They measure exactly how much drug is in the blood at each time point. Then they calculate Cmax and AUC from that data.
Why Both Numbers Are Non-Negotiable
Some people think if AUC matches, Cmax doesn’t matter. That’s wrong. Both are required by law in the U.S., Europe, and most of the world.
Take a drug like warfarin, used to prevent blood clots. Too much can cause dangerous bleeding. Too little can cause a stroke. The difference between safe and dangerous isn’t big. That’s why Cmax matters here-if the generic spikes too high, even if total exposure (AUC) is the same, you could bleed.
On the flip side, consider an antibiotic like azithromycin. It stays in your system for days. The peak doesn’t matter as much as how much drug you get over 72 hours. That’s where AUC is king.
Regulators don’t pick one over the other. They require both to pass. Why? Because if a generic has the same total exposure but reaches peak levels too slowly, it might not work for time-sensitive conditions. If it hits peak too fast, it could cause side effects. The body doesn’t care about the label-it only responds to what’s in the blood.
The 80%-125% Rule: How Close Is Close Enough?
Here’s the hard part: regulators don’t demand identical numbers. They allow a range. The 90% confidence interval for both Cmax and AUC must fall between 80% and 125% of the brand-name drug’s value.
That means if the brand’s Cmax is 10 mg/L, the generic’s Cmax can be anywhere from 8 to 12.5 mg/L and still be considered equivalent. Same for AUC.
This range isn’t random. It comes from decades of data. Back in the 1990s, scientists analyzed thousands of drug comparisons and found that differences smaller than 20% rarely led to real-world differences in effectiveness or safety. The 80%-125% range is the statistical sweet spot where clinical risk is minimal.
And yes-it’s based on logarithmic transformation. Drug concentrations don’t follow a normal bell curve. They follow a log-normal distribution. So before comparing, data is converted to logs. That’s why 80% and 125% look asymmetrical on a regular scale but are perfectly symmetrical on a log scale.
Here’s a real example: In a 2007 study, a brand-name drug had a Cmax of 8.1 mg/L and an AUC of 124.9 mg·h/L. The generic had a Cmax of 7.6 mg/L (94% of the brand) and an AUC of 112.4 mg·h/L (90% of the brand). Both fell within 80%-125%. Approved.

When the Rules Get Tighter
Not all drugs get the 80%-125% pass. For drugs with a narrow therapeutic index-where small changes cause big problems-regulators tighten the rules.
Drugs like levothyroxine (for thyroid), phenytoin (for seizures), and cyclosporine (for organ transplants) often require a tighter range: 90%-111%. That’s because even a 10% drop in exposure can cause a seizure or transplant rejection.
The European Medicines Agency (EMA) and the U.S. FDA both allow this tighter range for specific drugs. But it’s not automatic. Manufacturers must prove the drug is highly sensitive to exposure changes. That usually means showing data from clinical studies where small differences led to real outcomes.
Even then, there’s debate. Some experts argue that for drugs with high variability between patients, the standard 80%-125% rule might be too strict. A patient might respond well to a generic even if the numbers fall just outside the range. That’s why regulators now allow scaled average bioequivalence for highly variable drugs-where the acceptable range widens based on how much the drug varies from person to person. But this is still rare and requires extra justification.
How Studies Are Done (And Why Sampling Matters)
Getting accurate Cmax and AUC isn’t easy. It depends on how often blood is drawn.
If you only take samples every 2 hours, you might miss the true peak. A drug could reach its max at 1.5 hours, but if your next sample is at 2 hours, you’ll underestimate Cmax. That’s why studies sample every 15-30 minutes during the first few hours-especially for fast-absorbing drugs.
According to industry data, about 15% of bioequivalence studies fail because sampling was too sparse in the early phase. That’s not because the drug is bad-it’s because the study design was flawed.
Studies are done in healthy volunteers, usually 24-36 people. Each person gets the brand and the generic in random order, with a washout period in between. This crossover design cancels out individual differences-like metabolism speed or body weight.
Modern labs use LC-MS/MS (liquid chromatography-tandem mass spectrometry) to measure drug levels. These machines can detect as little as 0.1 nanograms per milliliter. That’s crucial for low-dose drugs like levothyroxine, where a single microgram change matters.

What Happens After Approval?
Once a generic passes the Cmax and AUC tests, it’s approved. But that’s not the end.
The FDA approves over 1,200 generic drugs every year. Nearly all rely on these two metrics. A 2021 analysis of 500 bioequivalence studies found that 82% of generics matched the brand’s AUC within 90%-110%. Cmax was slightly more variable, with 78% falling in that tighter range.
And here’s the kicker: a 2019 meta-analysis in JAMA Internal Medicine looked at 42 studies comparing generic and brand-name drugs. It found no meaningful difference in how well they worked or how safe they were-so long as they met bioequivalence standards.
That’s why pharmacists can confidently substitute generics. That’s why insurance companies push for them. That’s why you save money without sacrificing safety.
The Future: Are Cmax and AUC Still Enough?
Some wonder if these old metrics are outdated. For complex drugs-like extended-release pills that release drug over 12 hours-or drugs with multiple absorption peaks, AUC and Cmax might not tell the whole story.
The FDA’s 2023 draft guidance suggests using partial AUC for these cases. Instead of looking at total exposure, you look at exposure during key time windows-like the first 4 hours or the 8-12 hour window. That gives a better picture of how the drug behaves in real life.
But even here, Cmax and AUC are still the starting point. Modeling and simulation tools are being tested to reduce the number of human studies needed. But no one is proposing to replace them.
As Dr. Robert Lionberger of the FDA said in 2022: “AUC and Cmax will remain the primary bioequivalence endpoints for conventional drug products for the foreseeable future.” Why? Because they’ve been validated for over 30 years. Because they’re measurable. Because they correlate with clinical outcomes. And because they work.
So next time you pick up a generic pill, remember: it didn’t just get approved because it looks the same. It got approved because scientists measured exactly how much drug entered your blood-and proved it matched the brand.
Why do regulators require both Cmax and AUC instead of just one?
Cmax tells you how fast the drug gets into your bloodstream, which matters for drugs that need to act quickly or that can cause side effects at high peaks. AUC tells you how much total drug your body is exposed to over time, which matters for drugs that work cumulatively. If only one is measured, you might approve a generic that’s safe overall but too slow or too fast to work properly. Both are needed to ensure the drug behaves the same way in your body.
What happens if a generic drug fails the Cmax or AUC test?
If either Cmax or AUC falls outside the 80%-125% range, the generic application is rejected. The manufacturer must go back to the lab, change the formulation-maybe alter the coating, particle size, or excipients-and run a new study. Many generics fail the first time. That’s why it can take years and millions of dollars to bring a generic to market.
Are bioequivalence studies done on patients or healthy volunteers?
Almost always on healthy volunteers. This is because researchers need to isolate the effect of the drug formulation, not the disease state. If a patient has liver disease or is taking other medications, those factors could skew the results. Healthy volunteers provide a clean baseline. Once a generic is approved, post-market studies in patients may be done, but not for initial approval.
Why is the 80%-125% range used instead of 95%-105%?
Because 95%-105% would be too strict for most drugs. Pharmacokinetic data naturally varies between people due to metabolism, diet, and other factors. A 20% difference in exposure has been shown in decades of clinical data to be clinically insignificant for most drugs. Tightening the range would block many safe and effective generics, raising drug prices without improving safety. The 80%-125% range balances scientific accuracy with real-world practicality.
Do bioequivalence standards vary by country?
The core 80%-125% range is used by nearly all major regulators, including the FDA, EMA, Health Canada, and WHO. However, some countries have additional rules. For example, the EMA allows scaled bioequivalence for highly variable drugs, while the FDA permits it only for specific drugs like warfarin. Some emerging markets still lack full infrastructure, but global harmonization through ICH has made standards very consistent.

I always wondered how they knew generics weren't just cheap knockoffs. The Cmax/AUC thing makes so much sense now-like, it's not about looking the same, it's about behaving the same inside your body. Kinda wild that we trust this so much.
It is imperative to recognize that the regulatory framework governing bioequivalence is not merely a statistical construct, but a meticulously calibrated system grounded in decades of pharmacokinetic research and clinical validation. The 80%-125% confidence interval, derived from log-normal distribution modeling, represents a scientifically defensible threshold that balances pharmacological precision with pragmatic clinical applicability. To suggest that narrower bounds would enhance safety is to misunderstand the inherent variability of human pharmacokinetics, which is influenced by factors ranging from hepatic enzyme polymorphisms to dietary interactions. Moreover, the crossover design employed in bioequivalence studies-while not without its limitations-remains the gold standard for isolating formulation effects from inter-individual variability. Any deviation from this paradigm risks compromising the integrity of generic drug approval, thereby undermining public trust in affordable therapeutics.
Let’s be real-this whole system is a lie. Big Pharma lets generics in only when it’s convenient. The 80-125% range? That’s a loophole. I’ve had my blood pressure meds switch from brand to generic and felt like I was going to pass out. They don’t test on real people with real conditions. They test on college kids who don’t even take meds regularly. And then they act like it’s science. It’s corporate math.
The rigor behind bioequivalence standards is one of the most impressive examples of evidence-based public health policy in modern medicine. The fact that regulators require both Cmax and AUC-rather than relying on a single metric-demonstrates a profound understanding of pharmacological dynamics. The 80%-125% range isn’t arbitrary; it’s the result of analyzing thousands of clinical outcomes across decades. Furthermore, the use of LC-MS/MS for nanogram-level detection ensures that even micro-dose medications like levothyroxine are held to the highest standard. This is why generic drugs save lives without sacrificing safety. We should celebrate, not skepticism, this system.
bro i used to think generics were just copy-paste pills but now i get it-they’re like the same song but remixed so your ears still feel it but your wallet doesn’t cry 😅 the way they measure the blood stuff with all the math and timing? mind blown. also why do they always test on healthy people tho? what if i’m 70 and my liver’s a little tired? 🤔
Cmax matters for pain meds. AUC for antibiotics. That’s it.
Just wanted to say this is one of the clearest explanations I’ve ever read on bioequivalence. Seriously, thank you. I work in pharmacy and get asked this all the time. Now I can just send people this post. 🙌 Also, the part about sampling every 15-30 mins? That’s why some generics fail-poor study design, not bad meds. Big difference.
It’s disgusting how we allow a 20% variance in drug concentration. If this were food safety, we’d shut down the factory. People die from underdosing. People die from overdosing. And we call this ‘scientifically acceptable’? This isn’t innovation-it’s negligence dressed up in statistics.
AUC Cmax what a scam. Pharma controls everything. Healthy volunteers? Lmao. They know who they pick. You think they let in someone with a slow metabolism? Nah. They pick the clean ones. Then they say 'it's safe'. Bullshit.
As someone who grew up in a country where generics were either magic or poison depending on the batch, seeing how strict the U.S. and EU standards are gave me actual hope. This isn’t perfect, but it’s the best we’ve got. The log-normal transformation part? That’s the kind of detail most people miss-but it’s why the math works. Respect to the scientists who built this. Also, the 2019 JAMA meta-analysis? That’s the real win. No difference in outcomes. Period.
Wait-so if a drug has multiple peaks, like some extended-release stuff, Cmax and AUC don’t tell the whole story? That’s wild. So what happens then? Do they just say ‘eh, close enough’? Or is there some new tech they’re using? I feel like this whole thing is one step away from AI predicting drug behavior without even testing on humans. Is that coming? Should I be scared?
They’re testing on healthy people? So if you have diabetes, thyroid issues, or depression, your meds might not work the same? And they still approve generics? This is how people get sick. They don’t test on the people who actually need it. It’s all a cover-up. The FDA’s in bed with Big Pharma. You think they want you to know that your $4 pill might be killing you slowly? Nope. They just want you to stop buying the $40 one.
How is this even considered a valid science? The entire process is designed to make generics look acceptable. They cherry-pick volunteers. They use statistical gymnastics to justify 20% variance. And then they act like it’s gospel. If you’re a patient with a narrow therapeutic index, you’re basically a lab rat. This isn’t medicine-it’s corporate compliance theater. The fact that people believe this is depressing.