When you pick up a generic pill at the pharmacy in Berlin, Paris, or Warsaw, you might assume it’s the same everywhere. But behind that simple bottle lies a complex, fragmented, and rapidly changing regulatory system that determines when and where you can get affordable medicines. Across the European Union, generic drugs aren’t just approved the same way-they’re fought over, delayed, and strategically navigated through four different legal pathways, each with its own rules, costs, and bottlenecks. And in 2025, everything is shifting.
The Four Paths to Market: How Generics Get Approved in the EU
There’s no single route for a generic drug to reach patients in Europe. Instead, manufacturers must choose one of four approval systems, each with different timelines, costs, and outcomes.
The Centralized Procedure is the fastest way to get a generic approved across all 27 EU countries, plus Iceland, Liechtenstein, and Norway. It’s run by the European Medicines Agency (EMA), and if approved, the product can be sold everywhere at once. But it’s expensive-application fees alone start at €425,000, and total costs can hit €1.8 million when you factor in consultants, studies, and documentation. That’s why only about 15% of generic applications use this route. It’s reserved for high-value drugs with potential EU-wide sales over €250 million. Sandoz used it successfully for its version of Novartis’s Cosentyx, launching across Europe 11 months faster than through other methods.
The Mutual Recognition Procedure (MRP) is the most popular, used in 42% of cases. A company gets approval first in one country (the Reference Member State), then asks others to accept it. Sounds simple, right? Not really. Even though the technical review should take 90 days, delays are common. In 2023, Teva’s generic rosuvastatin got approved in Germany but waited 8.2 months before hitting Dutch and Belgian shelves because of pricing negotiations and local bureaucracy. The clock doesn’t stop until every country agrees.
The Decentralized Procedure (DCP) lets companies apply to multiple countries at the same time. No prior approval needed. But coordination is messy. The EMA’s own data shows 37% of DCP applications face delays longer than six months, mostly because Eastern European regulators interpret quality standards differently. One company reported that Germany demanded extra stability data on a polymorphic compound, while Poland accepted the same batch without issue. That inconsistency makes supply planning a nightmare.
The National Procedure is the slowest and most limited-only valid in one country. It’s used for just 5% of applications, usually when a company wants to target a single high-reimbursement market like France. But even there, it’s often slower than MRP. Accord Healthcare found it took 197 days to get approval in France via the national route, while the same drug got approved across five countries in 142 days using MRP.
What Makes a Generic ‘Equal’? The Science Behind Approval
Before any of these pathways even begin, the generic must prove it’s identical to the brand-name drug. Not just similar. Identical.
The EMA requires three things: same active ingredient, same dosage form (tablet, injection, inhaler), and proven bioequivalence. Bioequivalence means the body absorbs the generic drug at the same rate and extent as the original. That’s tested through clinical studies where volunteers take both versions, and blood samples are taken over time to measure concentration levels.
The gold standard? The 90% confidence interval for two key measurements-Cmax (peak concentration) and AUC (total exposure)-must fall between 80% and 125%. That’s not a suggestion. It’s a hard rule. And in 2025, the EMA updated its bioequivalence guidelines to require stricter protocols for complex generics like inhalers, patches, and injectables. Germany’s BfArM now demands extra pharmacodynamic studies for inhalers that other countries don’t require. That means a company might pass in Italy but fail in Germany, forcing them to retest or repackage.
The 2025 Pharma Package: A Major Reset
For years, the EU’s generic system was criticized for being slow and inconsistent. The 2025 Pharma Package-finalized on June 4, 2025-is the biggest overhaul in 20 years. It’s not just tweaking rules; it’s changing how the market works.
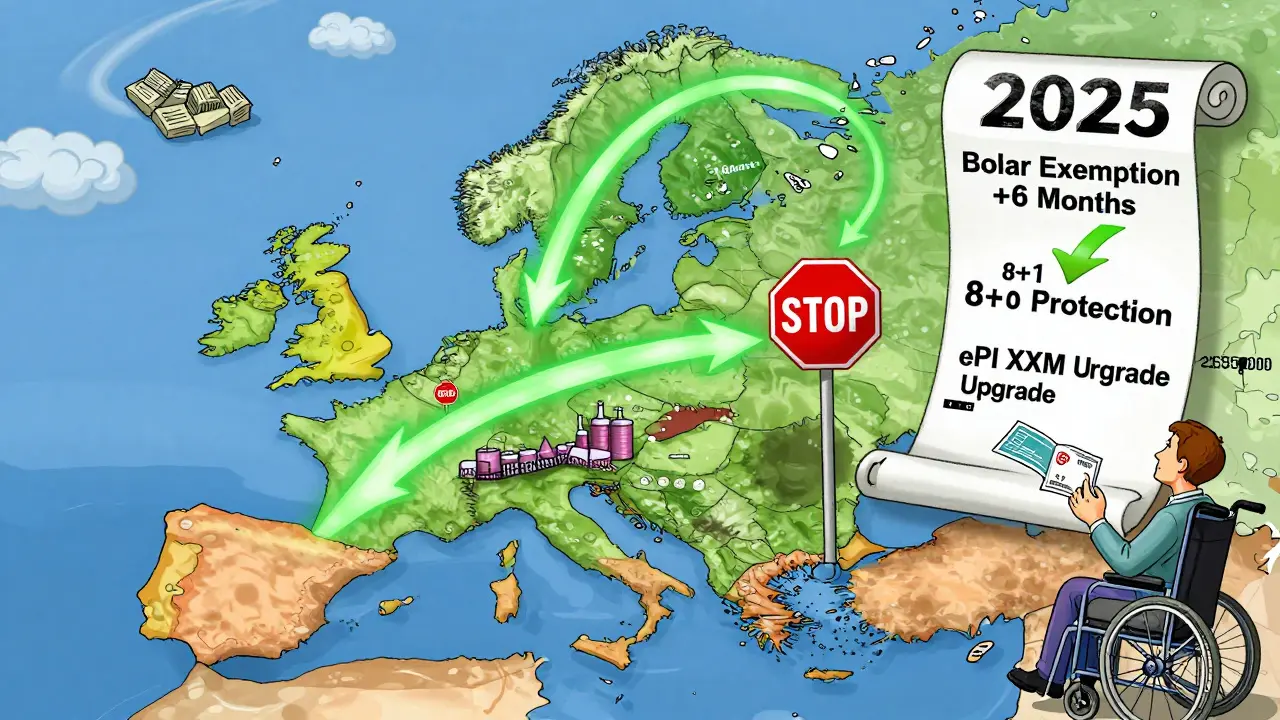
One big change: the Bolar exemption. Before, generic companies could only start pricing and reimbursement talks two months before a patent expired. Now, they can start six months early. That’s a game-changer. REMAP Consulting estimates this alone will speed up market entry by 4.3 months on average. It gives payers more leverage too-health systems can now negotiate prices before the generic even hits shelves, which could drop launch prices by 12-18%.
Another shift: Regulatory Data Protection is being shortened from 10 years to 8 years plus 1 year of market exclusivity (8+1). That means if a brand-name drug has 10 years of protection, the generic can enter after 9. It’s still enough to reward innovation, but it opens the door faster. For 78 high-value biologics in development, this could mean generics arrive years sooner than before.
But it’s not all smooth sailing. The new system introduces a €490 million sales threshold for “Transferable Exclusivity Vouchers”-a reward for companies that develop generics for rare diseases. Critics say this favors big players. Mid-sized firms might not reach that sales target, locking them out of incentives.
Who’s Winning and Who’s Struggling?
The EU generics market was worth €42.7 billion in 2024, growing 6.2% from the year before. But the winners aren’t evenly spread.
Indian manufacturers captured 38% of all EU generic approvals in 2024, up from 29% in 2020. Their low-cost production and focus on high-volume, low-margin drugs give them an edge. Meanwhile, European firms like Sandoz and Viatris hold 52% of the market-not because they’re cheaper, but because they play the long game. They use the Centralized Procedure to hit the whole market at once, avoiding the delays of national negotiations.
But even big players suffer. Mylan (now Viatris) reported in 2024 that MRP delays added €3.2 million in carrying costs per high-value launch. That’s not just paperwork-it’s warehouse space, interest on capital, and lost sales. And for smaller companies? The cost of compliance is crushing. The new requirement to submit electronic product information (ePI) in XML format by 2026 will cost €180,000-€250,000 per company to upgrade systems. Many can’t afford it.
Why Fragmentation Still Exists-and Why It Matters
Even with harmonized rules, national authorities still have final say on pricing, reimbursement, and sometimes even quality interpretation. That’s the real bottleneck.
A 2023 European Commission report found that, on average, it takes 11.3 months longer for a generic to become available in all EU countries than it does in the first country to approve it. That’s not just a delay-it’s a health equity issue. Patients in Portugal or Romania wait months longer than those in Germany or the Netherlands for the same medicine.
The 2025 Critical Medicines Act adds another layer: mandatory stockpiling of 200 essential generics. It sounds good-prevents shortages. But it also means manufacturers must meet new quality verification standards to qualify. That’s another hurdle for smaller firms.
And here’s the irony: the EU spends billions trying to harmonize drug approval, yet the biggest barrier isn’t science-it’s bureaucracy. One survey found 68% of generic companies say inconsistent national bioequivalence requirements are their biggest headache. The EMA sets the rules, but national agencies interpret them differently. A company might submit the same data to France and Poland and get two different responses.
What’s Next? The Road to 2028
By 2028, the EU expects generic prescriptions to rise from 65% to 69.2% of all prescriptions by volume. That’s good for patients. It’s also good for budgets.
The reforms are designed to cut the gap between U.S. and EU generic launch times-from 22.4 months down to something closer to Canada’s 8.7 months. The new obligation-to-supply mechanism, which forces manufacturers to keep adequate stock, could reduce medicine shortages by 35% by 2028.
But risks remain. Professor Panos Kanavos of LSE Health warns that the 1-year default market protection might discourage investment in complex generics-like biosimilars for rare conditions. If the profit window is too short, companies won’t bother. That could create new access gaps.
Meanwhile, the US-EU Framework Agreement, effective September 2025, may affect ingredient tariffs. No one knows yet how much that will raise costs, but for manufacturers already operating on thin margins, every extra cent counts.
What This Means for Patients and Providers
At the end of the day, the system’s complexity doesn’t matter to the person holding the prescription. They just want the medicine-affordably and on time.
For pharmacists, it means more questions: Why is this generic available here but not there? Why does this one cost more? For doctors, it means understanding which generics are truly interchangeable-and which ones might have hidden delays or formulation differences that affect patient outcomes.
For patients, the 2025 reforms should mean faster access to cheaper drugs. But only if the system actually works as intended. And that depends on whether national authorities finally stop treating EU rules as suggestions.
Generics are not a luxury. They’re the backbone of affordable healthcare in Europe. The regulatory framework was built to make them accessible. Now, it’s time to make sure it does.
How long does it take to get a generic drug approved in the EU?
Approval times vary by pathway. The Centralized Procedure takes about 210 days for assessment, with final approval in 67 days. The Mutual Recognition Procedure averages 132.7 days, and the Decentralized Procedure takes around 247 days. National procedures can take 180-240 days. Delays from national negotiations often add months beyond the official timeline.
What’s the difference between the Centralized and Mutual Recognition Procedures?
The Centralized Procedure gives approval across the entire EU at once through the EMA, but costs over €1.5 million. The Mutual Recognition Procedure starts with approval in one country, then asks others to accept it. It’s cheaper (€180K-€220K) but slower because each country can delay approval for non-scientific reasons like pricing or reimbursement.
Why do some generic drugs appear in some EU countries but not others?
Even after technical approval, a generic can’t be sold until it’s priced and reimbursed by each country’s health authority. Delays in pricing negotiations, differing reimbursement rules, or administrative backlogs can block availability-even if the drug is legally approved. This is why the same generic might be on shelves in Germany but not in Poland for months.
What changed in the EU’s 2025 Pharma Package for generics?
Three major changes: (1) The Bolar exemption now allows companies to start pricing talks 6 months before patent expiry (up from 2 months); (2) Regulatory Data Protection is reduced from 10 years to 8+1 years; and (3) A new obligation-to-supply rule requires manufacturers to maintain adequate stock of essential generics to prevent shortages.
Are Indian generic manufacturers dominating the EU market?
Yes. In 2024, Indian companies secured 38% of all EU generic approvals, up from 29% in 2020. They compete on low cost and high volume, especially for simple, high-demand generics. European firms still hold 52% of the market by value, largely because they use the Centralized Procedure for high-value drugs and have stronger distribution networks.
Do all EU countries accept the same bioequivalence data for generics?
Technically, yes-the EMA sets the standard. But in practice, no. Germany, for example, requires extra pharmacodynamic studies for inhalers. France demands pediatric formulation details. Poland and Romania sometimes have different interpretations of impurity limits. This inconsistency is the biggest operational challenge for generic manufacturers.

The bioequivalence standards are rock-solid, but the national-level interpretation chaos is absurd. I’ve seen the same batch cleared in Poland and rejected in Germany over minor polymorph variance reports. It’s not science-it’s administrative arbitrage.
Let me tell you-this is why American patients are confused when they travel. I worked in a pharmacy in Chicago, and we had patients bring in ‘European generics’ thinking they were ‘better.’ Nope. Same pill. Different paperwork. The EU’s system is a bureaucratic labyrinth designed by people who’ve never held a prescription.
From a pharmacoeconomic standpoint, the 8+1 RDP model represents a calibrated recalibration of incentive alignment-particularly for complex generics with high fixed costs. The Bolar exemption extension to six months is a non-trivial regulatory arbitrage lever, effectively compressing the time-to-market latency curve by ~130 days on average. However, the €490M threshold for transferable vouchers introduces a structural barrier for mid-tier manufacturers lacking scale economies in distribution and reimbursement lobbying.
So let me get this straight. India gets 38% of approvals because they’re cheap? And the EU is letting them in? This is why our drugs cost so much-because we’re outsourcing quality to countries that can’t even spell ‘pharmaceutical’ right. And now they’re getting subsidies? No. Just no.
As a Canadian observer deeply invested in transatlantic pharmaceutical equity, I find the fragmentation of national reimbursement protocols to be not merely inefficient, but ethically indefensible. The fact that a patient in Bucharest must wait 11.3 months longer than one in Berlin for an identical therapeutic agent constitutes a violation of the fundamental principle of health equity. The 2025 Pharma Package, while commendable in intent, fails to mandate binding harmonization of pricing timelines-a critical oversight.
INDIA IS STEALING OUR PHARMA MARKET!!! 🇮🇳💸 WHO LET THEM IN?!?!?!?! The EMA is a joke-why are we letting foreign labs make our life-saving pills?!?!?!? We need tariffs. We need bans. We need AMERICAN generics made by AMERICANS for AMERICANS!!! 🇺🇸💊 #MakeGenericsGreatAgain #EUWeakness
Centralized = expensive. MRP = slow. DCP = chaotic. National = pointless. The system is designed to fail. Only megacorps survive. Everyone else drowns in compliance costs. The 2025 reforms? Just rearranging deck chairs on the Titanic.
Interesting piece, though I wonder if the ePI mandate by 2026 will hit SMEs harder than anticipated. We’ve seen similar transitions in UK post-Brexit-costs ballooned, small firms exited. Maybe a phased rollout with subsidies would help? Just a thought.
Indian manufacturers are just exploiting loopholes. They don’t care about quality-they care about profit. And now they’re getting more approvals? 😒 This is why Europe’s healthcare is falling apart. No one’s checking the labs. No one’s looking at the batches. It’s a disaster waiting to happen. 💊☠️
Oh wow, so the EU spent 20 years building a system where a pill approved in France can’t be sold in Romania because someone in Bucharest ‘doesn’t like the color of the tablet.’ Brilliant. Just brilliant. Next they’ll require a notarized letter from the patient’s pet goldfish.
I appreciate how the 2025 reforms are trying to bridge gaps-Bolar extension, reduced exclusivity, supply mandates. But we need a unified digital gateway for regulatory submissions. Imagine one portal, one format, one review team. It’s technically possible. We’ve done it for VAT and customs. Why not meds? 🤝💊
This is why we can't have nice things. People are dying because some company in India made a pill that’s ‘close enough.’ That’s not medicine-that’s gambling with lives. And now you want to make it faster? No. We need more rules, not fewer.
Real talk: if you’ve ever waited months for a generic because of paperwork, you know this system is broken. But honestly? I’m glad someone’s finally trying to fix it. The Bolar change alone is huge. Let’s keep pushing-more transparency, less bureaucracy. Patients win when we cut the red tape.
It is of considerable interest to observe that the structural heterogeneity of national regulatory interpretations, particularly with regard to pharmacodynamic parameters for complex drug delivery systems, constitutes a non-trivial impediment to the harmonization of pharmaceutical access across the European Union. Furthermore, the differential application of impurity thresholds, while ostensibly grounded in localized risk assessments, inadvertently perpetuates disparities in therapeutic availability that are both statistically significant and ethically concerning. One might posit, therefore, that the implementation of a centralized, AI-driven adjudication layer for bioequivalence data submission could mitigate these inconsistencies-though such a proposal would necessitate robust data governance frameworks and cross-border legal consensus, both of which remain aspirational at present.