When your doctor hands you a prescription for a brand-name drug, you might not realize there’s a cheaper version sitting right next to it on the pharmacy shelf. Generic medications are just as safe and effective as their brand-name counterparts-but they can cost 80-85% less. In 2022, the average brand-name drug cost $765.09. The same drug as a generic? Just $15.23. That’s not a typo. If you’re paying full price for a brand, you could be throwing away hundreds-or even thousands-of dollars a year.
What Makes a Generic Drug Actually Work?
Not all generics are created equal in people’s minds, but they are in the eyes of the FDA. To get approved, a generic must have the same active ingredient, strength, dosage form, and route of administration as the brand-name drug. That means if your prescription is for 10 mg of lisinopril, the generic has to be exactly 10 mg of lisinopril. No more, no less.
The real test is bioequivalence. The FDA requires generics to deliver the same amount of medicine into your bloodstream within the same timeframe as the brand. The acceptable range? Between 80% and 125% of the brand’s absorption rate. That’s tight enough to ensure your blood pressure, cholesterol, or thyroid levels stay stable.
There’s one exception: drugs with a narrow therapeutic index-like warfarin, levothyroxine, or phenytoin. Small changes in how these drugs are absorbed can lead to serious side effects. Even though generics for these drugs are approved, your doctor might prefer you stick with one brand or generic version consistently. Always ask if your drug falls into this category.
How to Find Out If a Generic Exists for Your Drug
You don’t need to be a pharmacist or a scientist to check if a generic is available. Here’s how to do it in three simple steps.
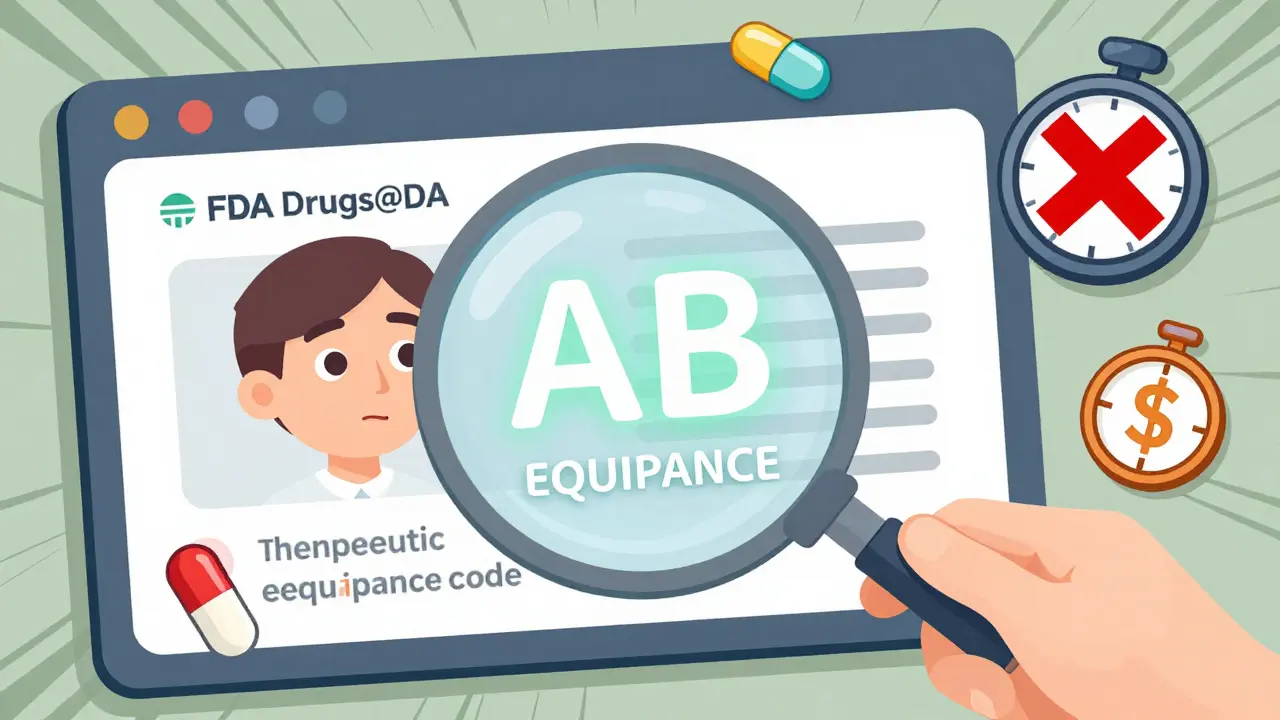
- Look up your drug in the FDA’s Drugs@FDA database. Go to Drugs@FDA and type in the brand name. Once you find the entry, scroll down to the "Therapeutic Equivalence Code." If it says "AB," that means the generic is approved as interchangeable. If it says "BX," the FDA has concerns about substitution. You’ll need to talk to your doctor before switching.
- Check the Orange Book. Officially called "Approved Drug Products with Therapeutic Equivalence Evaluations," this is the gold standard for determining if a generic is truly equivalent. The Orange Book uses those same AB/BX codes. You can search it for free on the FDA website. Many people skip this step because the interface looks outdated, but it’s the most reliable source.
- Ask your pharmacist. This is the fastest way. Pharmacists have real-time access to formulary databases like Medi-Span and First Databank. In a 2022 JAMA study, pharmacists correctly identified generic alternatives 98.7% of the time. Just say: "Is there a therapeutically equivalent generic available for this?" Don’t just ask, "Do you have a generic?"-that’s too vague. You want to make sure the substitute is approved as interchangeable.
What to Do When Your Insurance Automatically Switches You
Many insurance plans, especially Medicare Part D, automatically substitute generics unless your doctor specifically writes "Dispense as Written" on the prescription. That’s legal-and usually a good thing. But here’s the catch: you might not know it happened.
A 2023 study of Medicare users found that 41% of beneficiaries didn’t realize their brand-name drug had been switched to a generic. That’s not a problem if the generic works fine. But if you start feeling off-dizziness, nausea, unusual fatigue-it could be a formulation difference. Some generics use different inactive ingredients (fillers, dyes, coatings) that can affect how your body reacts, especially if you have allergies or sensitivities.
If you suspect a switch caused a problem, call your pharmacy. Ask them to confirm what version you got. Then call your doctor. You can request that your prescription be marked "Do Not Substitute"-but be prepared to explain why. Insurance companies often require prior authorization for brand-name drugs when a generic exists.
Why Some Drugs Still Don’t Have Generics
You might wonder: if generics are so cheap and safe, why aren’t they available for every drug?
The answer is patents. Brand-name companies hold patents that give them exclusive rights to sell a drug for up to 20 years from the date of filing. But that’s not the whole story. Many companies get patent extensions, legal loopholes, or exclusivity periods for new uses, formulations, or pediatric studies. As a result, about 10% of FDA-approved drugs still don’t have generic versions.
Complex drugs are another hurdle. Inhalers, injectables, and topical creams are harder to copy because small differences in how they’re made can change how the medicine works in your body. Biosimilars-generic versions of biologic drugs like Humira or Enbrel-are still rare. As of late 2023, only 38 had been approved in the U.S., compared to over 10,000 small-molecule generics.
Tools That Actually Help (and the Ones That Don’t)
You’ve probably seen apps like GoodRx, SingleCare, or Medfinder. They’re great for comparing prices, but they don’t tell you if a generic is therapeutically equivalent. GoodRx might show you a $5 generic version of a drug-but if it’s marked "BX" in the Orange Book, that doesn’t mean it’s safe to switch without your doctor’s approval.
Here’s what works:
- Pharmacy counters: The #1 way people find generics. 92% of CVS, Walgreens, and Rite Aid locations automatically flag generic options at checkout.
- FDA Drugs@FDA and Orange Book: Free, official, and accurate. Takes 8-12 minutes to learn, but you’ll never be misled again.
- Medicare Plan Finder: If you’re on Medicare, this tool shows you exactly which generics are covered under your plan-and at what price. Updated every October 15.
Here’s what doesn’t:
- Search engines like Google or Bing-results are often paid ads or outdated info.
- Commercial drug finders that charge $30 per search-no better than your pharmacist.
- Asking your doctor without checking the FDA first-many doctors don’t know the latest generic status.

Real Stories: How People Saved Hundreds a Year
A Reddit user in Ohio saved $1,200 a year just by asking for a generic version of metformin. The brand cost $140 a month. The generic? $4. Another person in Texas switched from a brand-name thyroid med to a generic and noticed no difference-except her out-of-pocket cost dropped from $98 to $12.
On TikTok, a nurse named @nurse_jen posted a 90-second video showing how to use the FDA’s Drugs@FDA tool. It got 2.4 million views. Comments flooded in: "I had no idea I was overpaying for 5 years," "I just saved my entire prescription budget," "Why didn’t anyone tell me this?"
The message is clear: you’re not being lazy if you ask. You’re being smart.
What to Do If the Generic Isn’t Working
Some people report side effects after switching to a generic-even when it’s rated "AB." That’s rare, but it happens. If you feel worse after the switch, don’t assume it’s all in your head.
Take notes: When did the symptoms start? What changed? Did you switch pharmacies? Did your insurance change your drug? Bring this info to your doctor. You can request to go back to the brand, or try a different generic. Not all generics are made the same. One manufacturer’s version might work better for you than another’s.
Also, check the ASHP Drug Shortages database. Sometimes, a generic isn’t working because it’s not available at all. If your drug is on shortage, your pharmacist might be forced to give you a brand-or a different generic with different ratings.
What’s Changing in 2024 and Beyond
Things are moving fast. Starting January 1, 2024, Medicare Part D plans must show real-time generic availability through the Medicare Plan Finder. That means you’ll know before you even walk into the pharmacy.
Electronic health record systems like Epic, used by over 250 million patients, are planning to integrate FDA therapeutic equivalence data directly into doctor’s prescription screens by late 2024. That means your doctor might get a pop-up saying: "Generic available. Cost: $15 vs. $750."
By 2028, 73% of the top-selling drugs in the U.S. will have generic versions. That’s good news for your wallet. But it also means more complexity-more choices, more codes, more things to check.
The bottom line? You have power. You don’t have to accept the first price you’re given. You don’t have to guess whether a generic is safe. You can find out-and save serious money.
How do I know if a generic drug is safe?
The FDA requires generics to meet the same strict standards as brand-name drugs. They must contain the same active ingredient, work the same way in your body, and be manufactured under the same quality controls. Look for the "AB" rating in the FDA’s Orange Book-that means it’s approved as interchangeable and safe to use.
Can I switch from a brand to a generic without telling my doctor?
In most cases, yes-your pharmacist can substitute a generic unless your doctor wrote "Dispense as Written." But if you’re on a drug with a narrow therapeutic index (like warfarin or levothyroxine), you should always check with your doctor first. Even small differences can matter.
Why does my generic look different from the brand?
By law, generics can’t look exactly like the brand-name drug-that’s to avoid trademark infringement. So the color, shape, or size might be different. But the active ingredient is the same. If you’re worried, check the drug name and strength on the label. That’s what matters.
Are all generics made in the same place?
No. Generics are made all over the world-including the U.S., India, China, and others. The FDA inspects all manufacturing facilities, whether they’re in Ohio or Hyderabad. As long as the drug is FDA-approved, the location doesn’t affect safety or effectiveness.
What if my insurance won’t cover the generic?
That’s unusual. Generics are almost always covered, and usually at the lowest tier. If your plan refuses to cover it, call your insurer. Ask for a formulary exception. If that doesn’t work, ask your pharmacist if there’s another generic version you can switch to. Sometimes, one manufacturer’s version is covered and another isn’t.
How often should I check if a new generic is available?
Every 6 to 12 months. New generics come out all the time-especially when patents expire. If you’re on a long-term medication, it’s worth checking again. You might be paying more than you need to.


bro i just asked my pharmacist for the generic and he gave me this weird blue pill that looked like it came from a sci-fi movie
turned out it was the same stuff as the brand but cheaper as hell
saved me 120 bucks a month on my blood pressure med
why do people still pay full price when the FDA says it's literally the same chemical
it's not magic it's math
It is indeed a matter of considerable importance that patients are made aware of the therapeutic equivalence between branded and generic pharmaceuticals. The regulatory framework established by the FDA ensures that such substitutions are both safe and efficacious. One ought to exercise due diligence in consulting the Orange Book and engaging with pharmacy professionals to ensure optimal outcomes.
so like... are you telling me the government lets companies in china make my heart pills and it's just as good?
my mind is blown
like if i bought a phone from china i'd be scared it'd explode
but my blood pressure med from a factory in hyderabad? cool
weird world man
I think about this every time I fill a prescription. It's not just about money-it’s about dignity. People are forced to choose between medicine and groceries, between feeding their kids and taking their insulin, because they don’t know they have a right to ask for the generic. The system doesn’t make it easy, but the fact that a 90-second TikTok video reached 2.4 million people? That’s hope. That’s power. That’s ordinary people reclaiming their health from a broken system that profits from ignorance. We’re not just saving dollars-we’re reclaiming agency. And that’s worth more than any brand name.
so like... i switched my thyroid med to generic and now i feel like a zombie
but also like i saved $86 a month
so... is my brain just broken or is the generic doing something weird?
also why does it look like a neon green pebble
my pharmacist just shrugged and said 'it's FDA approved'
cool i guess
why are we letting foreign countries make our medicine
if this was cars or guns we'd be in a war
but pills? oh sure, let china and india handle our health
the FDA says it's fine but i don't trust it
we need American-made meds or nothing
My mom switched to generic lisinopril and didn’t notice a thing. Then she saw the price and cried. Not from sadness-from relief. I wish more people knew this was an option. It’s not about trust. It’s about access. And if the science says it’s the same, then the only thing holding us back is marketing.
My grandmother in rural Mississippi didn’t know generics existed until her grandson showed her the Orange Book. She’d been paying $200 a month for a brand-name statin. The generic? $8. She said, ‘I didn’t think the government cared if I lived or not.’
It’s not just a drug. It’s a lifeline.
Generic saved me $900 last year. That’s a vacation. Or groceries. Or not choosing between meds and rent.
I used to think generics were ‘inferior’ until I started reading the FDA’s bioequivalence data. Turns out, the difference between 80% and 125% absorption is tighter than most people’s daily blood sugar fluctuations. We’re scared of the unknown, but the science isn’t. Maybe the real question isn’t whether generics work-it’s why we still believe the brand-name myth.
generic works fine till u switch to diff maker then u feel like u got poisoned
its all about the fillers
and why is the FDA letting this happen
its a scam