When your body overreacts to something harmless - a smell, a food, even stress - and you break out in hives, get dizzy, or feel like you’re having an allergic attack without a clear trigger, it might not be your allergies. It could be your mast cells going haywire.
What Are Mast Cells and Why Do They Matter?
Mast cells are immune cells that live in your skin, lungs, gut, and other places where your body meets the outside world. They were first spotted in 1878 by Paul Ehrlich, who noticed their granules looked like little bombs. He was right. These cells store powerful chemicals meant to defend you from parasites and pathogens. But when they activate at the wrong time, they turn into the source of chronic, confusing symptoms.
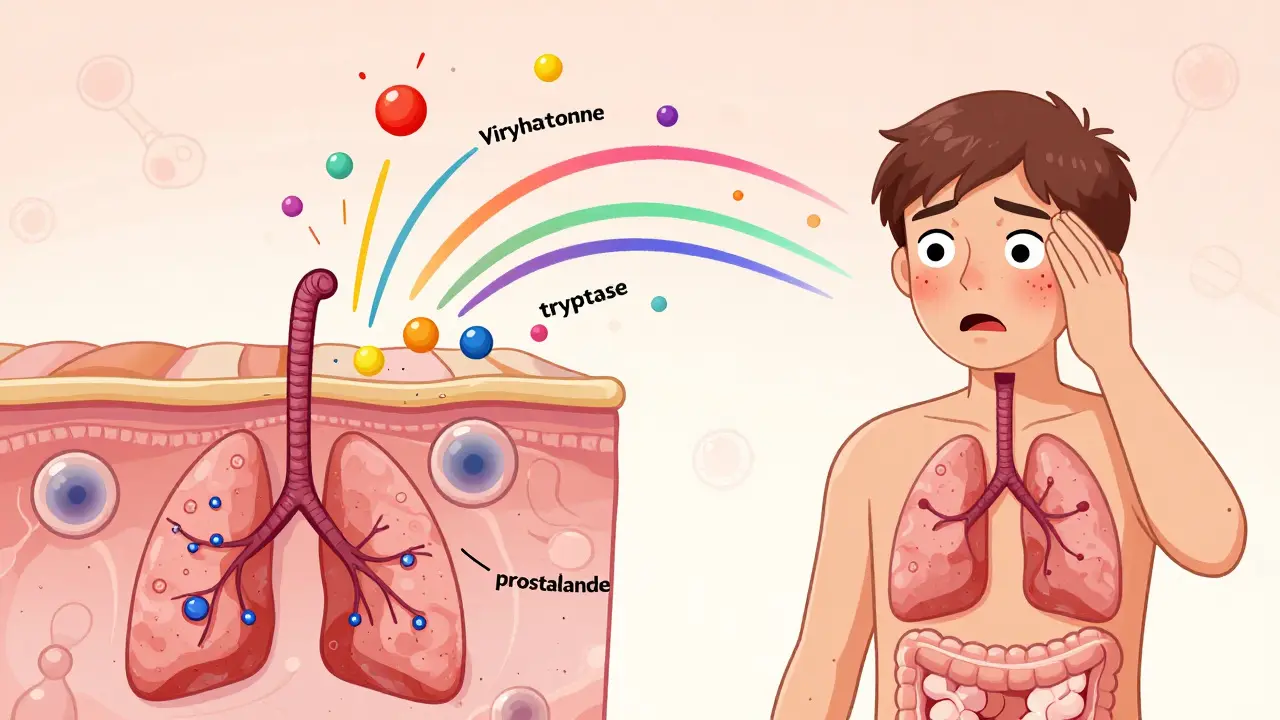
Unlike other immune cells that take hours to respond, mast cells react in seconds. When triggered, they dump out their contents - histamine, tryptase, prostaglandins, leukotrienes - into your tissues. That’s what causes flushing, itching, stomach cramps, brain fog, low blood pressure, and even anaphylaxis. The problem isn’t always an allergen. It can be heat, alcohol, NSAIDs, emotional stress, or even a change in barometric pressure.
For most people, this system works fine. But for an estimated 1 in 1,000 to 1 in 10,000 people, mast cells become overly sensitive. This is called Mast Cell Activation Syndrome (MCAS). It’s not rare - it’s just misunderstood. A 2019 study found that 17% of people with chronic hives have MCAS. Many spend years being told they have anxiety or IBS before someone connects the dots.
How Mast Cells Release Mediators - And Why It’s So Complex
Mast cells don’t just explode. They release chemicals in stages, like a carefully timed attack.
First come the pre-formed mediators - histamine, tryptase, chymase, and heparin - stored in granules inside the cell. These are released within seconds of activation. Histamine alone makes up 10-15% of the granule’s dry weight. Tryptase, which accounts for 20-30% of granule protein, is often measured in blood tests to confirm mast cell activation.
Then come the newly made mediators. Lipid-based molecules like prostaglandin D2 and leukotriene C4 are synthesized within minutes. These cause prolonged inflammation, bronchoconstriction, and pain. Hours later, cytokines like TNF-alpha and IL-6 flood the system, driving chronic fatigue, joint pain, and brain fog.
What triggers this? The most common pathway is IgE binding to the FcεRI receptor on mast cells - responsible for about 70% of allergic reactions. But non-IgE triggers are just as important. Things like:
- Complement proteins C3a and C5a (at concentrations as low as 10-100 nM)
- Gram-positive bacterial peptidoglycan (10-100 μg/ml)
- Neuropeptides like substance P
- Physical stimuli: heat, pressure, vibration
- Emotional stress - which activates the nervous system and directly signals mast cells
Some people have genetic mutations in genes like KIT, TPSAB1, or CBL that make their mast cells hyper-responsive. Around 30% of MCAS patients have one of these mutations, according to Dr. Cem Akin. That’s why two people exposed to the same trigger - say, red wine - can have wildly different reactions.
What Are Mast Cell Stabilizers and How Do They Work?
Mast cell stabilizers are drugs designed to stop the release of mediators before they happen. They don’t block histamine after it’s released - they prevent the explosion in the first place.
The most well-known is cromolyn sodium (disodium cromoglycate). Approved by the FDA in 1973 for asthma, it was later used for mastocytosis in 1996. It works by blocking calcium from entering mast cells. Without calcium, the granules can’t fuse with the cell membrane and release their contents.
Another option is ketotifen, approved in the U.S. in 1990. It does double duty: it stabilizes mast cells and also blocks H1 histamine receptors. Studies show it reduces MCAS symptoms in 50-70% of users at doses of 1-4 mg twice daily.
Unlike antihistamines - which only block one type of mediator - stabilizers prevent the release of dozens: histamine, tryptase, PGD2, LTC4, cytokines. That’s why they’re often more effective long-term, even if they don’t work fast.
But here’s the catch: they don’t work for acute attacks. You can’t take cromolyn when you’re having anaphylaxis. It’s a preventive tool. Think of it like wearing a seatbelt - it won’t save you in a crash, but it prevents injury if you’re driving safely.
How Effective Are They? Real Numbers, Real Experiences
Studies show mixed results - but real patients tell a clearer story.
A 2022 survey of 1,200 MCAS patients found that 87% felt better on mast cell stabilizers. But only 43% had complete symptom control. That means most still need other tools - antihistamines, low-histamine diets, stress management.
One patient, a 42-year-old woman, reported a 70% drop in anaphylactic episodes after starting cromolyn at 200 mg four times daily. But it took eight weeks to notice any change. That’s typical. These drugs build up slowly. You can’t expect overnight results.
Side effects are real. A 2021 Drug Safety study found 35% of cromolyn users had nausea or diarrhea. Fifteen% stopped taking it because of GI issues. The taste? A 2019 survey rated the oral solution at 2.1 out of 5. That’s why some kids need it through feeding tubes.
Compared to newer drugs like omalizumab (an anti-IgE biologic), stabilizers are less effective - 40-60% response rate versus 70-80% for biologics. But biologics cost tens of thousands a year. Cromolyn? Around $200 a month. Ketotifen? Even less.
How to Use Mast Cell Stabilizers Correctly
Starting cromolyn isn’t as simple as popping a pill.
Most doctors begin with 100 mg four times a day - 30 minutes before meals and at bedtime. That’s because food can interfere with absorption. After 2-4 weeks, if tolerated, the dose is slowly increased to 200-400 mg four times daily. Titration takes 4-6 weeks. Rushing it increases side effects.
How do you know if it’s working? Blood and urine tests help. Doctors track:
- 24-hour urinary methylhistamine (normal: under 1.3 mg)
- N-methyl-β-hexosaminidase (normal: under 1,000 ng/mg creatinine)
A successful response is a 30% or greater drop in these markers. Without testing, you’re guessing.
Also, track your triggers. The ‘mast cell trigger wheel’ - used by support groups - lists common ones: NSAIDs (68% of patients), alcohol (63%), heat (57%), stress (52%), and specific foods (49%). Avoiding triggers boosts stabilizer effectiveness.
The Bigger Picture: Where Is This Field Headed?
Mast cell biology is no longer a niche topic. The global market for mast cell stabilizers hit $1.2 billion in 2022 and is expected to double by 2030. Academic medical centers are opening dedicated mast cell clinics - up from 42% in 2015 to 78% today.
New drugs are coming. Avapritinib, approved by the FDA in 2023 for advanced systemic mastocytosis, targets the KIT D816V mutation. In trials, it cut mediator release by 60%. SYK kinase inhibitors are in Phase II trials and are showing 75% reduction in mediator release at 100 mg daily.
But for now, stabilizers remain the most accessible, affordable option for most MCAS patients. They won’t cure you. But they can give you back control - if you use them right.
Diagnosis Is the Biggest Hurdle
Most patients see 6-10 doctors over 3-5 years before getting diagnosed. They’re told they’re anxious. They’re given antidepressants. They’re told their IBS is “just stress.”
The diagnostic criteria are still debated. The International Consensus-Organized Nomenclature of Mast Cell Disorders (ICON-MCD) requires a serum tryptase increase of at least 20% plus 2 ng/mL above baseline. The AAAAI says clinical symptoms matter more - if you have multi-system symptoms, a positive response to stabilizers, and no other explanation, that’s enough.
Dr. Lawrence Afrin, a leading expert, says: “MCAS is a spectrum disorder. You don’t need a mutation or a spike in tryptase to have it. You need symptoms that match the biology.”
If you suspect MCAS, ask for:
- Baseline and acute tryptase (within 4 hours of a reaction)
- 24-hour urinary methylhistamine
- N-methyl-β-hexosaminidase
- Plasma prostaglandin D2 (if available)
And if your doctor says, “We don’t test for that,” find one who does. The Mast Cell Disease Society has a directory of 350 verified specialists.
Bottom Line: Stabilizers Aren’t Magic - But They’re Essential
Mast cell stabilizers won’t fix everything. You’ll still need to avoid triggers. You might still need antihistamines. You might still have bad days.
But if your body is constantly releasing histamine, tryptase, and cytokines for no good reason - and you’re tired of being told it’s all in your head - then stabilizers are the closest thing to a reset button you’ve got.
They’re slow. They’re messy. They don’t work for everyone. But for thousands of people, they’re the difference between being housebound and being able to eat dinner without fear.
Can mast cell stabilizers stop anaphylaxis?
No. Mast cell stabilizers like cromolyn and ketotifen are preventive - they stop mast cells from releasing mediators before they’re triggered. They do not work during an active reaction. For acute anaphylaxis, epinephrine is the only life-saving treatment. Always carry an epinephrine auto-injector if you have a history of severe reactions.
How long does it take for cromolyn to work?
It usually takes 4 to 8 weeks to see noticeable improvement. Some people notice small changes after 2-3 weeks, but full effect often requires 2 months of consistent use. This is because stabilizers need time to build up in tissues and modulate mast cell behavior - they don’t act like antihistamines, which block receptors immediately.
Are mast cell stabilizers safe long-term?
Yes. Cromolyn sodium and ketotifen have been used for decades with a strong safety profile. Long-term studies show no significant organ toxicity. The main side effects are gastrointestinal - nausea, diarrhea, cramping - which often improve with dose adjustment or taking the medication on an empty stomach. There is no evidence of dependence or withdrawal.
Do I need to avoid all triggers if I’m on a stabilizer?
Yes. Stabilizers reduce the likelihood of activation, but they don’t make you immune. Trigger avoidance is still the most effective way to prevent flare-ups. Studies show that patients who combine stabilizers with trigger management (like avoiding NSAIDs, alcohol, and heat) have significantly better outcomes than those who rely on medication alone.
Can I take mast cell stabilizers with antihistamines?
Absolutely. In fact, most MCAS patients take both. Stabilizers prevent mediator release; antihistamines block the effects of histamine after it’s released. H1 blockers (like cetirizine) and H2 blockers (like famotidine) are often used together. Ketotifen even combines both actions in one pill. This dual approach is standard in clinical practice.
Is MCAS the same as mastocytosis?
No. Mastocytosis is a rare condition where the body makes too many mast cells - often due to a KIT gene mutation - and these cells accumulate in tissues. MCAS involves normal or slightly increased numbers of mast cells that are overly reactive. You can have MCAS without mastocytosis, and vice versa. The symptoms overlap, but the underlying biology and diagnostic criteria are different.
What’s the best way to track my symptoms?
Keep a daily log: note food, stress levels, temperature, medications, and symptoms (hives, brain fog, GI issues, etc.). Use a simple app or notebook. Many patients find patterns - like flare-ups after eating certain foods or being in hot showers. This data helps doctors adjust treatment and identify your personal triggers. It’s the most valuable tool you have.

I used to think my random hives and brain fog were just stress... until I started tracking my triggers with a spreadsheet. Turns out, heat + red wine + my cat sneezing = full-on meltdown. Cromolyn didn't fix me overnight, but after 6 weeks? I ate pizza for the first time in 3 years without wanting to die. Still gotta avoid the gym on humid days, but I'm alive again.
I've been on ketotifen for 8 months. It's not glamorous, but it's the only thing that lets me leave the house without a plan B. The drowsiness? Worth it. I don't care if it's not FDA-approved for MCAS-my body doesn't care about labels.
This is all a Big Pharma scam to sell you pills you don't need. Mast cells are just your body trying to tell you you're eating too much sugar and watching too much Netflix. Go vegan. Stop using deodorant. Sleep on a crystal. You'll be fine. They don't want you to know this.
The pharmacokinetics of cromolyn sodium are fundamentally misunderstood by laypeople. The drug's bioavailability is severely compromised by gastric pH and food interactions. Without precise titration protocols and serial biomarker monitoring-methylhistamine, N-methyl-β-hexosaminidase, PGD2-any clinical improvement is anecdotal and statistically insignificant. You're not 'getting better,' you're experiencing placebo-driven regression to the mean.
Honestly, if you're relying on cromolyn because you won't just 'change your lifestyle,' that's the real problem. I used to have this too. Then I quit gluten, dairy, soy, caffeine, alcohol, and started doing yoga at 5am. No meds. No drama. Just clean living. If you can't do that, maybe your symptoms aren't MCAS-they're just laziness.
I dont know why ppl pay 200 a month for this when you can just drink apple cider vinegar and pray. Also why are we letting these docs test for 'methylhistamine' like its some secret code? We got real problems in this country and this is what we're spending money on? #AmericaFirst
The sample size in the 2022 survey is statistically underpowered. With n=1200, the confidence interval for 87% efficacy spans 84-90%, which does not meet the p<0.01 threshold required for clinical validation. Furthermore, self-reported outcomes are subject to recall bias and confirmation bias. Until double-blind, placebo-controlled trials are conducted with biomarker correlation, this remains anecdotal.
I love how this post breaks down the science without drowning you in jargon. I've been on ketotifen for a year now-still get bad days, but I can finally go to restaurants without panic. My kid even started eating broccoli again. Small wins, man. This isn't a cure, but it's a lifeline.
Oh wow, another person who thinks a $200 pill is going to fix their 'trigger wheel' and their inability to handle life. You know what really stabilizes mast cells? Therapy. And maybe not being a hypochondriac who thinks every burp is anaphylaxis.
I used to think MCAS was just my soul screaming because I hadn't cried in 7 years. Then I realized-mast cells don't care about your trauma. They just want you to feel the weight of every unprocessed emotion in your gut, your skin, your skull. The cromolyn? It's not medicine. It's a temporary ceasefire in a war your body never asked to fight. I weep for the ones who think they can 'fix' this with diet.
I've been tracking my symptoms for 18 months. My worst days? Always after hot showers, stress, and eating anything with MSG. I didn't even know MSG was in my 'healthy' soup. Now I read labels like a spy. It's boring, but it works. No meds needed.
Funny how we call this 'medicine' but the real treatment is just... not being a human in the 21st century. We're all just walking landmines of toxins, stress, and bad food. Stabilizers are like putting duct tape on a dam. It helps. But the river’s still rising.
I just want to say... 🤍 this post made me cry. Not because I'm sad. Because I finally feel seen. I thought I was broken. Turns out I'm just a mast cell with a soul. And I'm not alone.